Update: COVID-19 Among Workers in Meat and Poultry Processing Facilities ― United States, April–May 2020 Early Release / July 7, 2020 / 69
Michelle A. Waltenburg, DVM1,2; Tristan Victoroff, MPH1; Charles E. Rose, PhD1; Marilee Butterfield3; Rachel H. Jervis, MPH4; Kristen M. Fedak, PhD4; Julie A. Gabel, DVM5; Amanda Feldpausch, MPH5; Eileen M. Dunne, PhD1,2,6; Connie Austin, DVM7; Farah S. Ahmed, PhD8; Sheri Tubach, MPH8; Charles Rhea, MPH9; Anna Krueger, MS10; David A. Crum, DVM11; Johanna Vostok, MPH12; Michael J. Moore, MS12; George Turabelidze, MD13; Derry Stover, MPH14; Matthew Donahue, MD1,2,14; Karen Edge, MPH15; Bernadette Gutierrez15; Kelly E. Kline, MPH16; Nichole Martz17; James C. Rajotte, MS18; Ernest Julian, PhD18; Abdoulaye Diedhiou, MD19; Rachel Radcliffe, DVM19; Joshua L. Clayton, PhD20; Dustin Ortbahn, MPH20; Jason Cummins, MPH21; Bree Barbeau, MPH22; Julia Murphy, DVM23; Brandy Darby, DVM23; Nicholas R. Graff, MPH24; Tia K. H. Dostal, MPH24; Ian W. Pray, PhD1,2,25; Courtney Tillman, MPH26; Michelle M. Dittrich, MPH1; Gail Burns-Grant1; Sooji Lee, MSPH1; Alisa Spieckerman, MPH1; Kashif Iqbal, MPH1; Sean M. Griffing, PhD1; Alicia Lawson, MPH1; Hugh M. Mainzer, DVM1; Andreea E. Bealle, MPH1; Erika Edding1; Kathryn E. Arnold, MD1; Tomas Rodriguez, MA1; Sarah Merkle, MPH1; Kristen Pettrone, MD1,2; Karen Schlanger, PhD1; Kristin LaBar, MPH1; Kate Hendricks, MD1; Arielle Lasry, PhD1; Vikram Krishnasamy, MD1; Henry T. Walke, MD1; Dale A. Rose, PhD1; Margaret A. Honein, PhD1; COVID-19 Response Team (View author affiliations)
View suggested citation Summary What is already known about this topic?
COVID-19 outbreaks among meat and poultry processing facility workers can rapidly affect large numbers of persons.
What is added by this report?
Among 23 states reporting COVID-19 outbreaks in meat and poultry processing facilities, 16,233 cases in 239 facilities occurred, including 86 (0.5%) COVID-19–related deaths. Among cases with race/ethnicity reported, 87% occurred among racial or ethnic minorities. Commonly implemented interventions included worker screening, source control measures (universal face coverings), engineering controls (physical barriers), and infection prevention measures (additional hand hygiene stations).
What are the implications for public health practice?
Targeted workplace interventions and prevention efforts that are appropriately tailored to the groups most affected by COVID-19 are critical to reducing both COVID-19–associated occupational risk and health disparities among vulnerable populations.
Article Metrics Altmetric: Article has an altmetric score of 694 News (9) Twitter (825) Facebook (3) Reddit (1) Citations: Views: Views equals page views plus PDF downloads
Metric Details On This Page Discussion Acknowledgments Figure Tables Table 1
Table 2
References Related Materials PDF pdf icon[199K] The figure describes COVID-19 cases among workers in meat and poultry processing facilities and ways to reduce occupational risk.
Meat and poultry processing facilities face distinctive challenges in the control of infectious diseases, including coronavirus disease 2019 (COVID-19) (1). COVID-19 outbreaks among meat and poultry processing facility workers can rapidly affect large numbers of persons. Assessment of COVID-19 cases among workers in 115 meat and poultry processing facilities through April 27, 2020, documented 4,913 cases and 20 deaths reported by 19 states (1). This report provides updated aggregate data from states regarding the number of meat and poultry processing facilities affected by COVID-19, the number and demographic characteristics of affected workers, and the number of COVID-19–associated deaths among workers, as well as descriptions of interventions and prevention efforts at these facilities. Aggregate data on confirmed COVID-19 cases and deaths among workers identified and reported through May 31, 2020, were obtained from 239 affected facilities (those with a laboratory-confirmed COVID-19 case in one or more workers) in 23 states.* COVID-19 was confirmed in 16,233 workers, including 86 COVID-19–related deaths. Among 14 states reporting the total number of workers in affected meat and poultry processing facilities (112,616), COVID-19 was diagnosed in 9.1% of workers. Among 9,919 (61%) cases in 21 states with reported race/ethnicity, 87% occurred among racial and ethnic minority workers. Commonly reported interventions and prevention efforts at facilities included implementing worker temperature or symptom screening and COVID-19 education, mandating face coverings, adding hand hygiene stations, and adding physical barriers between workers. Targeted workplace interventions and prevention efforts that are appropriately tailored to the groups most affected by COVID-19 are critical to reducing both COVID-19–associated occupational risk and health disparities among vulnerable populations. Implementation of these interventions and prevention efforts† across meat and poultry processing facilities nationally could help protect workers in this critical infrastructure industry.
Distinctive factors that increase meat and poultry processing workers’ risk for exposure to SARS-CoV-2, the virus that causes COVID-19, include prolonged close workplace contact with coworkers (within 6 feet for ≥15 minutes) for long time periods (8–12 hour shifts), shared work spaces, shared transportation to and from the workplace, congregate housing, and frequent community contact with fellow workers. Many of these factors might also contribute to ongoing community transmission (1). To better understand the effect of COVID-19 on workers in these facilities nationwide, on June 6, 2020, CDC requested that state health departments report aggregate surveillance data through May 31, 2020, for workers in all meat and poultry processing facilities affected by COVID-19, including 1) the number and type of such facilities that had reported at least one confirmed COVID-19 case among workers, 2) the total number of workers in affected facilities, 3) the number of workers with laboratory-confirmed COVID-19, and 4) the number of COVID-19–related worker deaths. States reported COVID-19 cases determined by the Council of State and Territorial Epidemiologists confirmed case definition.§ States were asked to report demographic characteristics and symptom status of workers with COVID-19. Testing strategies and methods for collecting symptom data varied by workplace. Proportional distributions for demographic characteristics and symptom status were calculated for cases among workers in 21 states after excluding missing and unknown values; data were missing for sex in 25% of reports, age in 24%, race/ethnicity in 39%, and symptom status in 37%. States also provided information (from direct observation or from management at affected facilities) regarding specified interventions and prevention efforts that were implemented. A random-effects logistic regression model was used to obtain an estimate of the pooled proportion of asymptomatic (SARS-CoV-2 detected but symptoms never develop) or presymptomatic (SARS-CoV-2 detected before symptom onset) infections at the time of testing among workers who had positive SARS-CoV-2 test results. Five states provided prevalence data from facility-wide testing of 5,572 workers in seven facilities. Modeling was conducted and 95% confidence intervals (CIs) were calculated, with facilities treated as the random effect, using SAS software (version 9.4; SAS Institute).
Twenty-eight (56%) of 50 states responded, including 23 (82%) that reported at least one confirmed COVID-19 case among meat and poultry processing workers. Overall, 239 facilities reported 16,233 COVID-19 cases and 86 COVID-19–related deaths among workers (Table 1). The median number of affected facilities per state was seven (interquartile range = 3–14). Among 14 states reporting the total number of workers in affected facilities, 9.1% of 112,616 workers received diagnoses of COVID-19. The percentage of workers with COVID-19 ranged from 3.1% to 24.5% per facility.
Twenty-one states provided information on demographic characteristics and symptom status of workers with COVID-19. Among the 12,100 (75%) and 12,365 (76%) patients with information on sex and age, 7,288 (60%) cases occurred among males, and 5,741 (46%) were aged 40–59 years, respectively (Figure). Among the 9,919 (61%) cases with race/ethnicity reported, 5,584 (56%) were in Hispanics, 1,842 (19%) in non-Hispanic blacks (blacks), 1,332 (13%) in non-Hispanic whites (whites), and 1,161 (12%) in Asians. Symptom status was reported for 10,284 (63%) cases; among these, 9,072 (88%) workers were symptomatic, and 1,212 (12%) were asymptomatic or presymptomatic.
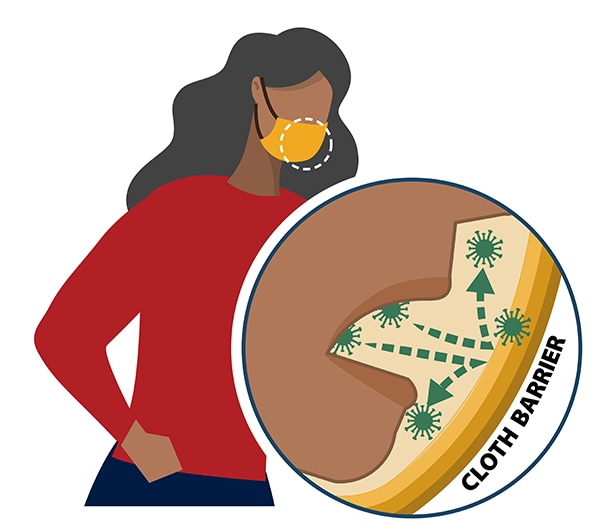
Among 239 facilities reporting cases, information on interventions and prevention efforts was available for 111 (46%) facilities from 14 states. Overall, 89 (80%) facilities reported screening workers on entry, 86 (77%) required all workers to wear face coverings, 72 (65%) increased the availability of hand hygiene stations, 70 (63%) educated workers on community spread, and 69 (62%) installed physical barriers between workers (Table 2). Forty-one (37%) of 111 facilities offered testing for SARS-CoV-2 to workers; 24 (22%) reported closing temporarily as an intervention measure.
Among seven facilities that implemented facility-wide testing, the crude prevalence of asymptomatic or presymptomatic infections among 5,572 workers who had positive SARS-CoV-2 test results was 14.4%. The pooled prevalence estimated from the model for the proportion of asymptomatic or presymptomatic infections among workers in meat and poultry processing facilities was 11.2% (95% CI = 0.9%–23.1%).
Top
Discussion The animal slaughtering and processing industry employs an estimated 525,000 workers in approximately 3,500 facilities nationwide (2,3). Combining data on workers with COVID-19 and COVID-19–related deaths identified and reported through May 31 from 23 states (16,233 cases; 86 deaths) with data from an earlier assessment through April 27 (1,125 cases; five deaths) (1) that included data from six states that did not contribute updated data to this report,¶ at least 17,358 cases and 91 COVID-19–related deaths have occurred among U.S. meat and poultry processing workers.
The effects of COVID-19 on racial and ethnic minority groups are not yet fully understood; however, current data indicate a disproportionate burden of illness and death among these populations (4,5). Among animal slaughtering and processing workers from the 21 states included in this report whose race/ethnicity were known, approximately 39% were white, 30% were Hispanic, 25% were black, and 6% were Asian.** However, among 9,919 workers with COVID-19 with race/ethnicity reported, approximately 56% were Hispanic, 19% were black, 13% were white, and 12% were Asian, suggesting that Hispanic and Asian workers might be disproportionately affected by COVID-19 in this workplace setting. Ongoing efforts to reduce incidence and better understand the effects of COVID-19 on the health of racial and ethnic minorities are important to ensure that workplace-specific prevention strategies and intervention messages are tailored to those groups most affected by COVID-19.
The proportion of asymptomatic or presymptomatic SARS-CoV-2 infections identified in investigations of COVID-19 outbreaks in other high-density settings has ranged from 19% to 88% (6,7). Among cases in workers with known symptom status in this report, 12% of patients were asymptomatic or presymptomatic; however, not all facilities performed facility-wide testing, during which these infections are more likely to be identified. Consequently, many asymptomatic and presymptomatic infections in the overall workforce might have gone unrecognized, and the approximations for disease prevalence in this report might underestimate SARS-CoV-2 infections. Recently derived estimates of the total proportion of asymptomatic and presymptomatic infections from data on COVID-19 investigations among cruise ship passengers and evacuees from Wuhan, China, ranged from 17.9% to 30.8%, respectively (8,9). The estimated proportion of asymptomatic and presymptomatic infections among meat and poultry processing workers (11.2%) is lower than are previously reported estimates and should be reevaluated as more comprehensive facility-wide testing data are reported.
In coordination with state and local health agencies, many meat and poultry processing facilities have implemented interventions to reduce transmission or prevent ongoing exposure within the workplace, including offering testing to workers.†† Expanding interventions across these facilities nationwide might help protect workers in this industry. Recognizing the interaction of workplace and community, many facilities have also educated workers about strategies for reducing transmission of COVID-19 outside the workplace.§§
The findings in this report are subject to at least seven limitations. First, only 28 of 50 states responded; 23 states with COVID-19 cases among meat and poultry processing facility workers submitted data for this report. In addition, only facilities with at least one laboratory-confirmed case of COVID-19 among workers were included. Thus, these results might not be representative of all U.S. meat and poultry processing facilities and workers. Second, delays in identifying workplace outbreaks and linking cases or deaths to outbreaks might have resulted in an underestimation of the number of affected facilities and cases among workers. Third, data were not reported on variations in testing availability and practices, which might influence the number of cases reported. Fourth, industry data were used for race/ethnicity comparisons; demographic characteristics of total worker populations in affected facilities were not available, limiting the ability to quantify the degree to which some racial and ethnic minority groups might be disproportionately affected by COVID-19 in this industry. Reported frequencies of demographic and symptom data likely underestimate the actual prevalence because of missing data, which limits the conclusions that can be drawn from descriptive analyses. Fifth, information on interventions and prevention efforts was available for a subset of affected facilities and therefore might not be generalizable to all facilities. Information was subject to self-report by facility management, and all available intervention efforts might not have been captured. Further evaluation of the extent of control measures and timing of implementations is needed to assess effectiveness of control measures. Sixth, symptom data collected at facility-wide testing was self-reported and might have been influenced by the presence of employers. Finally, workers in this industry are members of their local communities, and their source of exposure and infection could not be determined; for those living in communities experiencing widespread transmission, exposure might have occurred within the surrounding community as well as at the worksite.
High population-density workplace settings such as meat and poultry processing facilities present ongoing challenges to preventing and reducing the risk for SARS-CoV-2 transmission. Collaborative implementation of interventions and prevention efforts, which might include comprehensive testing strategies, could help reduce COVID-19–associated occupational risk. Targeted, workplace-specific prevention strategies are critical to reducing COVID-19–associated health disparities among vulnerable populations Lessons learned from investigating outbreaks of COVID-19 in meat and poultry processing facilities could inform investigations in other food production and agriculture workplaces to help prevent and reduce COVID-19 transmission among all workers in these essential industries.
Top
Acknowledgments State and local health departments in affected communities; affected facilities; CDC COVID-19 Emergency Response Health Department Task Force field team deployers; Julia Banks, Betsy Bertelsen, Elyse Bevers, Renee Canady, Kris Carter, Alyssa Carlson, Alex Cox, Meredith Davis, Chas DeBolt, Zachary Doobovsky, Marcia Goldoft, Anna Halloran, Lea Hammer, Michelle Holshue, Logan Hudson, Stephanie Kellner, Jennifer Lam, Shawn Magee, Laina Mitchell, Ellie Morgan, Sarah Murray, Laura Newman, Amal Patel, Chelsea Pugh, Jonathan Richardson, Tim Roth, Katrina Saphrey, Betsy Schroeder, Melissa Sixberry, Lisa Sollot, Alison Stargel.
COVID-19 Response Team Keith Amoroso, Rhode Island Department of Health; Yvette Diallo, CDC; Kathie Fazekas, CDC; Phillip J. Finley, CDC; Jennifer Fuld, CDC; Jodie L. Guest, Emory University, Atlanta, Georgia; Jocelyn J. Herstein, Global Center for Health Security University of Nebraska Medical Center; Erin D. Kennedy, CDC; James V. Lawler, Global Center for Health Security University of Nebraska Medical Center; John J. Lowe, Global Center for Health Security University of Nebraska Medical Center; Alexander Neifert, Rhode Island Department of Health; Michelle M. Schwedhelm, Global Center for Health Security; Nebraska Medicine; Jonathan M. Steinberg, South Dakota Department of Health; Douglas B. Trout, CDC; Max Zarate-Bermudez, CDC.
Top
Corresponding author: Michelle A. Waltenburg, mwaltenburg@cdc.gov.
Top
1CDC COVID-19 Emergency Response; 2Epidemic Intelligence Service, CDC; 3Arizona Department of Health Services; 4Colorado Department of Public Health and Environment; 5Georgia Department of Public Health; 6Idaho Department of Health and Welfare; 7Illinois Department of Public Health; 8Kansas Department of Health and Environment; 9Kentucky Department for Public Health; 10Maine Center for Disease Control and Prevention; 11Maryland Department of Health; 12Massachusetts Department of Public Health; 13Missouri Department of Health and Senior Services; 14Nebraska Department of Health and Human Services; 15New Mexico Department of Health; 16Pennslyvania Department of Health; 17Pennsylvania Department of Agriculture; 18Rhode Island Department of Health; 19South Carolina Department of Health and Environmental Control; 20South Dakota Department of Health; 21Tennessee Department of Health; 22Utah Department of Health; 23Virginia Department of Health; 24Washington State Department of Health; 25Wisconsin Department of Health Services; 26Wyoming Department of Health.
Top
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Top
* Arizona, Colorado, Georgia, Idaho, Illinois, Kansas, Kentucky, Maine, Maryland, Massachusetts, Missouri, Nebraska, New Mexico, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Utah, Virginia, Washington, Wisconsin, and Wyoming.
† https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/meat-poultry-processing-workers-employers.html.
§ https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/.
¶ Delaware, Iowa, Mississippi, North Carolina, Ohio, and Texas did not contribute data to this report.
** Data produced for 21 of 23 states (Colorado and Kansas did not provide information on demographic characteristics and symptom status of cases) using the Bureau of Census American Community Survey (CMS) Public Use Microdata Sample (PUMS) query tool (https://www.census.gov/programs-surveys/acs/data/pums.htmlexternal icon). Employment summaries were based on the American Community Survey 2014–2018 5-year PUMS estimates. Workforce estimates for Bureau of Census Industry Code 1180 (Animal Slaughtering and Processing) were tabulated by race/ethnicity using recoded detailed Hispanic origin and race.
References
snip...see full text;
It is Time to Address Airborne Transmission of COVID-19
Subject: GAO COVID-19 : Opportunities to Improve Federal Response and Recovery Efforts
 https://homelandprepnews.com/stories/51539-gao-highlights-opportunities-to-improve-federal-covid-19-response-recovery-efforts/
https://homelandprepnews.com/stories/51539-gao-highlights-opportunities-to-improve-federal-covid-19-response-recovery-efforts/
ACCEPTED MANUSCRIPT
It is Time to Address Airborne Transmission of COVID-19
Lidia Morawska, Donald K Milton
Clinical Infectious Diseases, ciaa939, https://doi.org/10.1093/cid/ciaa939
Commentary
We appeal to the medical community and to the relevant national and international bodies to recognize the potential for airborne spread of COVID-19. There is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale), and we are advocating for the use of preventive measures to mitigate this route of airborne transmission.
snip...
We are concerned that the lack of recognition of the risk of airborne transmission of COVID-19 and the lack of clear recommendations on the control measures against the airborne virus will have significant consequences: people may think that they are fully protected by adhering to the current recommendations, but in fact, additional airborne interventions are needed for further reduction of infection risk.
This matter is of heightened significance now, when countries are re-opening following lockdowns - bringing people back to workplaces and students back to schools, colleges, and universities. We hope that our statement will raise awareness that airborne transmission of COVID-19 is a real risk and that control measures, as outlined above, must be added to the other precautions taken, to reduce the severity of the pandemic and save lives.
It is Time to Address Airborne Transmission of COVID-19
TRUMP COVID VIRUS 2020
Subject: GAO COVID-19 : Opportunities to Improve Federal Response and Recovery Efforts
GAO highlights opportunities to improve federal COVID-19 response, recovery efforts

© Shutterstock
In accordance with the CARES Act containing a provision for the Government Accountability Office (GAO) to report on ongoing COVID-19 monitoring and oversight efforts, six recommendations have been issued.
The GAO indicated its recent analysis examined key actions the federal government has taken to address the pandemic and evolving lessons learned relevant to the nation’s response to pandemics.
Per the GAO, four relief laws were enacted as of June 2020 in response to COVID-19, including the CARES Act, in March 2020, with the measures having appropriated $2.6 trillion across the government.
The Paycheck Protection Program (PPP); Economic Stabilization and Assistance to Distressed Sectors; unemployment insurance; economic impact payments; Public Health and Social Services Emergency Fund; and Coronavirus Relief Fund accounted for 86 percent of the appropriations, the GAO indicated.
GAO identified several challenges related to the federal response to the crisis, officials said, noting the Centers for Disease Control and Prevention (CDC) reported incomplete and inconsistent data from state and jurisdictional health departments on the amount of viral testing occurring nationwide, making it more difficult to track and know the number of infections, mitigate their effects, and inform decisions on reopening communities; the nationwide need for critical supplies to respond to COVID-19 quickly exceeded the quantity of supplies contained in the Strategic National Stockpile; PPP borrowers and lenders raised several questions about the program and eligibility criteria, but after efforts to address the concerns, questions and confusion remained; and the Department of Labor currently has no mechanism in place to capture information in real-time about unemployment insurance claimants who may receive wages paid from PPP loan proceeds.
The GAO has offered the following recommendations, including urging Congress to take legislative action to require the Secretary of Transportation to work with relevant agencies and stakeholders, to develop a national aviation preparedness plan to ensure safeguards are in place to limit the spread of communicable disease threats from abroad while at the same time minimizing any unnecessary interference with travel and trade; provide agencies access to the Social Security Administration’s more complete set of death data and require that the Department of the Treasury consistently use it; and as a means of ensuring federal funding is targeted and timely, the GAO has urged Congress to use the agency’s Federal Medical Assistance Percentage formula for any future changes to the Federal Medical Assistance Percentage during the current or any future economic downturn.
Executive Action recommendations issued by the GAO include the Secretary of Labor immediately providing information to state unemployment agencies specifically addressing Paycheck Protection Program loans and the risk of improper payments associated with the loans; the Commissioner of Internal Revenue should consider cost-effective options for notifying ineligible recipients on how to return payments; and the Administrator of the Small Business Administration should develop and implement plans to identify and respond to risks in the Paycheck Protection Program to ensure program integrity, achieve program effectiveness and address potential fraud.
Energy and Commerce Chairman Frank Pallone, Jr. (D-NJ) said the GAO’s report provided a detailed accounting of the Trump Administration’s handling of the coronavirus pandemic.
“The report finds routine inconsistencies in the Administration’s testing data, perpetual shortages of critical supplies, and poorly coordinated response efforts that allowed the virus to spread at alarming rates,” Pallone said in response to the report. “The GAO’s findings echo concerns we heard from governors at a hearing earlier this month about how the federal government has hampered state efforts to deal with shortages of critical supplies. It raises questions about allocating supplies from the Strategic National Stockpile and how it will ensure the Stockpile is replenished to continue fighting COVID-19 infections.”
GAO COVID-19 : Opportunities to Improve Federal Response and Recovery Efforts
Report to the Congress
GAO-20-625; PUBLISHED: JUN 25, 2020 . PUBLICLY RELEASED:JUN 25, 2020
tRump and people of color, and mandated death by executive order?
Original Investigation
June 8, 2020
JAMA. Published online June 8, 2020. doi:10.1001/jama.2020.10369
Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2
Elizabeth Whittaker, MD1,2; Alasdair Bamford, MD3,4; Julia Kenny, MD5,6; et al
Key Points Question What are the clinical and laboratory characteristics of critically ill children who developed an inflammatory multisystem syndrome during the coronavirus disease 2019 pandemic?
Findings This case series included 58 hospitalized children, a subset of whom required intensive care, and met definitional criteria for pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (PIMS-TS), including fever, inflammation, and organ dysfunction. Of these children, all had fever and nonspecific symptoms, such as abdominal pain (31 [53%]), rash (30 [52%]), and conjunctival injection (26 [45%]); 29 (50%) developed shock and required inotropic support or fluid resuscitation; 13 (22%) met diagnostic criteria for Kawasaki disease; and 8 (14%) had coronary artery dilatation or aneurysms. Some clinical and laboratory characteristics had important differences compared with Kawasaki disease, Kawasaki disease shock syndrome, and toxic shock syndrome.
Meaning These findings help characterize the clinical features of hospitalized, seriously ill children with PIMS-TS and provide insights into this apparently novel syndrome.
Abstract Importance In communities with high rates of coronavirus disease 2019, reports have emerged of children with an unusual syndrome of fever and inflammation.
Objectives To describe the clinical and laboratory characteristics of hospitalized children who met criteria for the pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (PIMS-TS) and compare these characteristics with other pediatric inflammatory disorders.
Design, Setting, and Participants Case series of 58 children from 8 hospitals in England admitted between March 23 and May 16, 2020, with persistent fever and laboratory evidence of inflammation meeting published definitions for PIMS-TS. The final date of follow-up was May 22, 2020. Clinical and laboratory characteristics were abstracted by medical record review, and were compared with clinical characteristics of patients with Kawasaki disease (KD) (n = 1132), KD shock syndrome (n = 45), and toxic shock syndrome (n = 37) who had been admitted to hospitals in Europe and the US from 2002 to 2019.
Exposures Signs and symptoms and laboratory and imaging findings of children who met definitional criteria for PIMS-TS from the UK, the US, and World Health Organization.
Main Outcomes and Measures Clinical, laboratory, and imaging characteristics of children meeting definitional criteria for PIMS-TS, and comparison with the characteristics of other pediatric inflammatory disorders.
Results Fifty-eight children (median age, 9 years [interquartile range {IQR}, 5.7-14]; 33 girls [57%]) were identified who met the criteria for PIMS-TS. Results from SARS-CoV-2 polymerase chain reaction tests were positive in 15 of 58 patients (26%) and SARS-CoV-2 IgG test results were positive in 40 of 46 (87%). In total, 45 of 58 patients (78%) had evidence of current or prior SARS-CoV-2 infection. All children presented with fever and nonspecific symptoms, including vomiting (26/58 [45%]), abdominal pain (31/58 [53%]), and diarrhea (30/58 [52%]). Rash was present in 30 of 58 (52%), and conjunctival injection in 26 of 58 (45%) cases. Laboratory evaluation was consistent with marked inflammation, for example, C-reactive protein (229 mg/L [IQR, 156-338], assessed in 58 of 58) and ferritin (610 μg/L [IQR, 359-1280], assessed in 53 of 58). Of the 58 children, 29 developed shock (with biochemical evidence of myocardial dysfunction) and required inotropic support and fluid resuscitation (including 23/29 [79%] who received mechanical ventilation); 13 met the American Heart Association definition of KD, and 23 had fever and inflammation without features of shock or KD. Eight patients (14%) developed coronary artery dilatation or aneurysm. Comparison of PIMS-TS with KD and with KD shock syndrome showed differences in clinical and laboratory features, including older age (median age, 9 years [IQR, 5.7-14] vs 2.7 years [IQR, 1.4-4.7] and 3.8 years [IQR, 0.2-18], respectively), and greater elevation of inflammatory markers such as C-reactive protein (median, 229 mg/L [IQR 156-338] vs 67 mg/L [IQR, 40-150 mg/L] and 193 mg/L [IQR, 83-237], respectively).
Conclusions and Relevance In this case series of hospitalized children who met criteria for PIMS-TS, there was a wide spectrum of presenting signs and symptoms and disease severity, ranging from fever and inflammation to myocardial injury, shock, and development of coronary artery aneurysms. The comparison with patients with KD and KD shock syndrome provides insights into this syndrome, and suggests this disorder differs from other pediatric inflammatory entities.
Snip...
Since the first reports of an unusual inflammatory illness in children that emerged in the months following the onset of COVID-19, there have been additional reports from many countries of children with fever and inflammation, for which no cause could be identified, first in health alerts and web exchanges between professional groups, and then in case reports and small case series in rapid publications.2-4 As these cases have emerged in temporal association with the pandemic, a link with SARS-CoV-2 is likely.
COVID-19 AND CHILDREN
Published Online May 6, 2020 https://doi.org/10.1016/ S0140-6736(20)31094-1
Hyperinflammatory shock in children during COVID-19 pandemic
South Thames Retrieval Service in London, UK, provides paediatric intensive care support and retrieval to 2 million children in South East England. During a period of 10 days in mid-April, 2020, we noted an unprecedented cluster of eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome,1 or toxic shock syndrome (typical number is one or two children per week). This case cluster formed the basis of a national alert. All children were previously fit and well. Six of the children were of AfroCaribbean descent, and five of the children were boys. All children except one were well above the 75th centile for weight. Four children had known family exposure to coronavirus disease 2019 (COVID-19). Demographics, clinical findings, imaging findings, treatment, and outcome for this cluster of eight children are shown in the table.
Clinical presentations were similar, with unrelenting fever (38–40°C), variable rash, conjunctivitis, peripheral oedema, and generalised extremity pain with significant gastrointestinal symptoms. All progressed to warm, vasoplegic shock, refractory to volume resuscitation and eventually requiring noradrenaline and milrinone for haemodynamic support. Most of the children had no significant respiratory involvement, although seven of the children required mechanical ventilation for cardiovascular stabilisation. Other notable features (besides persistent fever and rash) included development of small pleural, pericardial, and ascitic effusions, suggestive of a diffuse inflammatory process.
All children tested negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on bronchoalveolar lavage or nasopharyngeal aspirates. Despite being critically unwell, with laboratory evidence of infection or inflammation3 including elevated concentrations of C-reactive protein, procalcitonin, ferritin, triglycerides, and D-dimers, no pathological organism was identified in seven of the children. Adenovirus and enterovirus were isolated in one child.
Baseline electrocardiograms were non-specific; however, a common echocardiographic finding was echobright coronary vessels (appendix), which progressed to giant coronary aneurysm in one patient within a week of discharge from paediatric intensive care (appendix). One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct. The myocardial involvement2 in this syndrome is evidenced by very elevated cardiac enzymes during the course of illness.
All children were given intravenous immunoglobulin (2 g/kg) in the first 24 h, and antibiotic cover including ceftriaxone and clindamycin. Subsequently, six children have been given 50 mg/kg aspirin. All of the children were discharged from PICU after 4–6 days. Since discharge, two of the children have tested positive for SARSCoV-2 (including the child who died, in whom SARS-CoV-2 was detected post mortem). All children are receiving ongoing surveillance for coronary abnormalities.
We suggest that this clinical picture represents a new phenomenon affecting previously asymptomatic children with SARS-CoV-2 infection manifesting as a hyperinflammatory syndrome with multiorgan involvement similar to Kawasaki disease shock syndrome. The multifaceted nature of the disease course underlines the need for multispecialty input (intensive care, cardiology, infectious diseases, immunology, and rheumatology).
The intention of this Correspondence is to bring this subset of children to the attention of the wider paediatric community and to optimise early recognition and management. As this Correspondence goes to press, 1 week after the initial submission, the Evelina London Children’s Hospital paediatric intensive care unit has managed more than 20 children with similar clinical presentation, the first ten of whom tested positive for antibody (including the original eight children in the cohort described above).
We declare no competing interests.
*Shelley Riphagen, Xabier Gomez, Carmen Gonzalez-Martinez, Nick Wilkinson, Paraskevi Theocharis shelley.riphagen@gstt.nhs.uk
South Thames Retrieval Service for Children, Evelina London Children’s Hospital Paediatric Intensive Care Unit, London SE1 7EH, UK (SR, XG); and Evelina London Children’s Hospital, London, UK (CG-M, NW, PT)
2 New York Boys Die Of Multi-System Inflammatory Syndrome Affecting Children Amid Coronavirus Pandemic
May 8, 2020 at 11:41 pmFiled Under:Coronavirus, COVID-19, Health, Jessica Layton, Local TV, multi-symptom inflammatory syndrome, New York, Tony Aiello, Valhalla, Westchester County
VALHALLA, N.Y. (CBSNewYork) — A Westchester County boy has died after coming down with an illness affecting dozens of children in New York State.
The 7-year-old boy died late last week at Maria Fareri Children’s Hospital in Valhalla. Dr. Michael Gewitz said he suffered neurological complications from what is now called pediatric multi-system inflammatory syndrome.
Health officials said there have been 73 suspected cases of the illness statewide and investigators are doing a deep dive into the circumstances.
Gov. Andrew Cuomo shared an update Friday, announcing the death of a 5-year-old boy, who CBS2 later confirmed died at Mount Sinai Kravis Children’s Hospital.
“Right now we have a new issue that we’re looking at, which is something we’re just investigating now, but, while rare, we’re seeing some cases where children affected with the COVID virus can become ill with symptoms similar to the Kawasaki disease or Toxic Shock-like syndrome that literally causes inflammation in their blood vessels,” Cuomo said. “This past Thursday, a 5-year-old boy passed away from COVID-related complications, and the State Department of Health is investigating several other cases that presents similar circumstances.”
The hospital said in part, “We must emphasize that based on what we know thus far, it appears to be a very rare condition.”
WATCH: Gov. Cuomo Warns About New Disease Affecting Children Amid Pandemic
It’s still unclear exactly how the syndrome relates to the coronavirus.
The Westchester boy tested positive for COVID-19 antibodies, meaning he was previously infected and had recovered, CBS2’s Tony Aiello reported.
“And we know that in some of the households parents or grandparents or others were diagnosed with COVID and were actually on the record being positive, and apparently the children did not develop symptoms until two to four days before presenting to the hospital for treatment,” said Dr. Dial Hewlett of the Westchester County Department of Health.
“This is very serious. The disease can be fatal, and we want to make sure everyone in Westchester County is aware to be on the lookout for symptoms that may lead to this,” County Executive George Latimer added.
Web Extra: Health Advisory On Pediatric Multi-System Inflammatory Syndrome
Seek care immediately if a child has:
Prolonged fever (more than 5 days)
Difficulty feeding (infants) or is too sick to drink fluids
Severe abdominal pain, diarrhea, or vomiting
Change in skin color – becoming pale, patchy, and/or blue Trouble breathing or is breathing very quickly
Racing heart or chest pain
Decreased amount or frequency of urine Lethargy, irritability, or confusion
“So this is every parent’s nightmare, right? That your child may actually be affected by this virus. But it’s something we have to consider seriously now,” Gov. Cuomo said.
In New Jersey, a 4-year-old child with underlying health issues has also died. It’s unclear if he was affected by the inflammatory syndrome, but there are a handful of other suspected cases in Garden State kids.
“It’s a virus that’s proving to be extremely challenging at every level,” Gov. Phil Murphy said.
Dr. Gewitz said while COVID-19 is likely to infect a large number of children, “most of whom, at least many, are totally asymptomatic. This particular complication is relatively infrequent, unusual.”
SARS-CoV-2 Infection in Children
Of the 1391 children assessed and tested from January 28 through February 26, 2020, a total of 171 (12.3%) were confirmed to have SARS-CoV-2 infection. Demographic data and clinical features are summarized in Table 1. (Details of the laboratory and radiologic findings are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.) The median age of the infected children was 6.7 years. Fever was present in 41.5% of the children at any time during the illness. Other common signs and symptoms included cough and pharyngeal erythema. A total of 27 patients (15.8%) did not have any symptoms of infection or radiologic features of pneumonia. A total of 12 patients had radiologic features of pneumonia but did not have any symptoms of infection. During the course of hospitalization, 3 patients required intensive care support and invasive mechanical ventilation; all had coexisting conditions (hydronephrosis, leukemia [for which the patient was receiving maintenance chemotherapy], and intussusception). Lymphopenia (lymphocyte count, <1 .2="" 10-month-old="" 149="" 2020="" 21="" 4="" 6="" 8="" a="" admission.="" after="" and="" as="" been="" bilateral="" child="" common="" condition="" death.="" died="" discharged="" div="" failure="" finding="" from="" general="" ground-glass="" had="" have="" hospital.="" in="" intussusception="" liter="" march="" most="" multiorgan="" of="" one="" opacity="" patients="" per="" present="" radiologic="" stable="" the="" there="" total="" wards="" was="" weeks="" were="" with="">
This report describes a spectrum of illness from SARS-CoV-2 infection in children. In contrast with infected adults, most infected children appear to have a milder clinical course. Asymptomatic infections were not uncommon.2 Determination of the transmission potential of these asymptomatic patients is important for guiding the development of measures to control the ongoing pandemic.
Two COVID-19 infected children, aged 12 and 13, die in Belgium and UK
By Alasdair Sandford with AFP, AP • last updated: 01/04/2020
A health worker in the intensive care ward observes a COVID-19 patient at a hospital in Belgium, March 27, 2020. (AP Photo/Francisco Seco, File)
A 12-year-old girl in Belgium and a 13-year old boy in the UK infected with the novel coronavirus have died, authorities said.
They are believed to be the youngest victims of the disease in their respective countries.
The 12-year-old girl's death was announced during the daily news conference given by Belgium's health service, at the end of its regular update on casualty figures and hospitalisations.
“It's an emotionally difficult moment because it involves a child, and it has also upset the medical and scientific community,” said spokesman Dr Emmanuel André, visibly upset.
"We are thinking of her family and friends. It's a very rare event, but one which devastates us."
Another spokesman added that the child had had a fever for three days and had tested positive for the coronavirus. No other details were given of the girl's background.
Until now the youngest person to die from the virus in Belgium was a 30-year-old female nurse, according to Belgian media.
Just a few hours later, London's King's College Hospital announced that a 13-year-old COVID-19 patient had also died.
"Sadly, a 13-year old boy who tested positive for COVID-19 has passed away, and our thoughts and condolences are with the family at this time," a Trust spokesperson said in a statement.
"The death has been referred to the Coroner," it added.
An appeal posted on the GoFunMe crowdfunding platform by Madinah College, named him as Ismail and said that he didn't have "any pre-existing health conditions.
"Sadly he died without any family members close by due to the highly infectious nature of COVID-19," it added.
Ismail is believed the be the youngest victim of the disease in the UK.
Last week French authorities said a 16-year-old girl had died at a children's hospital in Paris. The death of the teenager, identified as Julie A. and described as otherwise healthy, has provoked strong emotions in France.
Coronavirus in France: healthy 16 year-old dies of COVID-19
Deaths from COVID-19 among people so young are exceptional. Health authorities have said previously that serious cases of the illness -- although predominant in older and more vulnerable age groups -- can occur in adults of any age.
Last weekend the US state of Illinois announced the death of an infant under one year old who had tested positive for coronavirus. The cause of death was being investigated. Medical reports on cases in China have documented the death of a 10-month-old baby and a 14-year-old boy.
A recent US study by the Centers for Disease Control and Prevention (CDC) of 2,500 patients found no cases of deaths among people aged under 19. But it did find that people of all ages were liable to become seriously ill: more than a third of those hospitalised were aged between 20 and 54.
Coronavirus in Europe: Latest numbers on COVID-19 cases and deaths
The Belgian girl's death was included among the latest national figures released on Tuesday, confirming nearly 200 more deaths since the previous update. More than 700 people in the country have died from coronavirus since the outbreak began.
Hospitals in three regions have been particularly badly affected, the authorities say -- around Brussels, in Limburg in eastern Flanders, and in Hainaut in Wallonia to the west.
With 12,775 confirmed COVID-19 cases as of Tuesday, Belgium has the 10th highest number of infections among countries worldwide, according to data compiled by the US Johns Hopkins University Coronavirus Resource Center.
June 15, 2020
Gary L. Disbrow Ph.D. Deputy Assistant Secretary Director, Medical Countermeasure Programs Biomedical Advanced Research and Development Authority (BARDA) Office of Assistant Secretary for Preparedness and Response (ASPR) U.S. Department of Health and Human Services (HHS) 330 Independence Ave, S.W., Room 640G Washington, D.C. 20201
Dear Dr. Disbrow:
This letter is in response to your request, dated today, that the Food and Drug Administration (FDA) revoke the Emergency Use Authorization (EUA) for emergency use of oral formulations of chloroquine phosphate (CQ) and hydroxychloroquine sulfate (HCQ) to be distributed from the Strategic National Stockpile (SNS) issued on March 28, 2020. Like BARDA’s earlier request to FDA to issue the EUA, BARDA’s request to revoke the EUA is part of a collaborative, USGinteragency effort to rapidly respond to this continuously evolving public health emergency. Today’s request to revoke is based on new information, including clinical trial data results, that have led BARDA to conclude that this drug may not be effective to treat COVID-19 [Coronavirus Disease 2019] and that the drug’s potential benefits for such use do not outweigh its known and potential risks.
The authorization of a product for emergency use under section 564 of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. 360bbb-3) may, pursuant to section 564(g)(2) of the Act, be revised or revoked when the criteria under section 564(b)(1) of the Act no longer exist, the criteria under section 564(c) of the Act for issuance of such authorization are no longer met, or other circumstances make such revision or revocation appropriate to protect the public health or safety.
FDA has determined that the criteria under section 564(c) of the Act for issuance of the EUA referenced above are no longer met. Under section 564(c)(2) of the Act, an EUA may be issued only if FDA concludes “that, based on the totality of scientific evidence available to the Secretary, including data from adequate and well-controlled clinical trials, if available, it is reasonable to believe that: (A) the product may be effective in diagnosing, treating, or preventing—(i) such disease or condition [….]; and (B) the known and potential benefits of the product, when used to diagnose, prevent, or treat such disease or condition, outweigh the known and potential risks of the product […].”
As explained in the attached memorandum, based on a review of new information and a reevaluation of information available at the time the EUA was issued, FDA now concludes that these criteria are no longer met. The bases for this decision include the following:
FDA REVOKES Emergency Use Authorization (EUA) for emergency use of oral formulations of chloroquine phosphate (CQ) and hydroxychloroquine sulfate (HCQ)
As explained in the attached memorandum, based on a review of new information and a reevaluation of information available at the time the EUA was issued, FDA now concludes that these criteria are no longer met. The bases for this decision include the following:
THURSDAY, JUNE 11, 2020
The effect of large-scale anti-contagion policies on the COVID-19 pandemic
SUNDAY, MAY 17, 2020
CORONAVIRUS IN TEXAS, More than 700 new cases of coronavirus meatpacking plants Amarillo region, 11 county deaths connected to Long Term Care Facility at Texas City
CORONAVIRUS IN TEXAS
SATURDAY, MAY 9, 2020
Covid-19 Mortality, Crunching the Numbers, Children, The Jungle 1906 to 2020
REPORT
Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2
View ORCID ProfileJianzhong Shi1,*, View ORCID ProfileZhiyuan Wen1,*, View ORCID ProfileGongxun Zhong1,*, View ORCID ProfileHuanliang Yang1,*, View ORCID ProfileChong Wang1,*, View ORCID ProfileBaoying Huang2,*, Renqiang Liu1, Xijun He3, Lei Shuai1, Ziruo Sun1, Yubo Zhao1, View ORCID ProfilePeipei Liu2, Libin Liang1, Pengfei Cui1, Jinliang Wang1, View ORCID ProfileXianfeng Zhang3, Yuntao Guan3, View ORCID ProfileWenjie Tan2, View ORCID ProfileGuizhen Wu2,†, View ORCID ProfileHualan Chen1,†, View ORCID ProfileZhigao Bu1,3,† See all authors and affiliations
Science 29 May 2020: Vol. 368, Issue 6494, pp. 1016-1020 DOI: 10.1126/science.abb7015 Article Figures & Data Info & Metrics eLetters PDF Alternative hosts and model animals The severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) pandemic may have originated in bats, but how it made its way into humans is unknown. Because of its zoonotic origins, SARS-CoV-2 is unlikely to exclusively infect humans, so it would be valuable to have an animal model for drug and vaccine development. Shi et al. tested ferrets, as well as livestock and companion animals of humans, for their susceptibility to SARS-CoV-2 (see the Perspective by Lakdawala and Menachery). The authors found that SARS-CoV-2 infects the upper respiratory tracts of ferrets but is poorly transmissible between individuals. In cats, the virus replicated in the nose and throat and caused inflammatory pathology deeper in the respiratory tract, and airborne transmission did occur between pairs of cats. Dogs appeared not to support viral replication well and had low susceptibility to the virus, and pigs, chickens, and ducks were not susceptible to SARS-CoV-2.
Science, this issue p. 1016; see also p. 942
Abstract
Severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) causes the infectious disease COVID-19 (coronavirus disease 2019), which was first reported in Wuhan, China, in December 2019. Despite extensive efforts to control the disease, COVID-19 has now spread to more than 100 countries and caused a global pandemic. SARS-CoV-2 is thought to have originated in bats; however, the intermediate animal sources of the virus are unknown. In this study, we investigated the susceptibility of ferrets and animals in close contact with humans to SARS-CoV-2. We found that SARS-CoV-2 replicates poorly in dogs, pigs, chickens, and ducks, but ferrets and cats are permissive to infection. Additionally, cats are susceptible to airborne transmission. Our study provides insights into the animal models for SARS-CoV-2 and animal management for COVID-19 control.
snip...
In summary, we found that ferrets and cats are highly susceptible to SARS-CoV-2; dogs have low susceptibility; and pigs, chickens, and ducks are not susceptible to the virus. Unlike influenza viruses and the other SARS-coronavirus known to infect humans (SARS-CoV-1), which replicate in both the upper and lower respiratory tract of ferrets (20, 22–24, 26, 27), SARS-CoV-2 replicates only in the nasal turbinate, soft palate, and tonsils of ferrets. SARS-CoV-2 may also replicate in the digestive tract, as viral RNA was detected in the rectal swabs of the virus-infected ferrets, but virus was not detected in lung lobes, even after the ferrets were intratracheally inoculated with the virus. It remains unclear whether the virus causes more severe disease in male ferrets than in female ferrets, as has been observed among humans (13, 28).
Several studies have reported that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as its receptor to enter cells (3, 29–31). ACE2 is mainly expressed in type II pneumocytes and serous epithelial cells of tracheo-bronchial submucosal glands in ferrets (25). Ferrets and cats differ by only two amino acids in the SARS-CoV-2 spike-contacting regions of ACE2 (table S1); therefore, the underlying mechanism that prevents the replication of SARS-CoV-2 in the lower respiratory tract of ferrets remains to be investigated. The fact that SARS-CoV-2 replicates efficiently in the upper respiratory tract of ferrets makes them a candidate animal model for evaluating the efficacy of antiviral drugs or vaccines against COVID-19.
The cats we used in this study were outbred and were susceptible to SARS-CoV-2, which replicated efficiently and was transmissible to naïve cats. Cats in Wuhan have been reported to be seropositive for SARS-CoV-2 (32). Surveillance for SARS-CoV-2 in cats should be considered as an adjunct to elimination of COVID-19 in humans.
SUNDAY, MAY 17, 2020
CORONAVIRUS IN TEXAS, More than 700 new cases of coronavirus meatpacking plants Amarillo region, 11 county deaths connected to Long Term Care Facility at Texas City
We’re going to win so much, you’re going to be so sick and tired of winning, you’re going to come to me and go ‘Please, please, we can’t win anymore.’ You’ve heard this one. You’ll say ‘Please, Mr. President, we beg you sir, we don’t want to win anymore. It’s too much. It’s not fair to everybody else.’” Trump said.
Trump gop maga, “get back to work now” like leading sheep to slaughter...tss
WEDNESDAY, APRIL 29, 2020
President Donald J. Trump signed an Executive Order to keep meat and poultry processing facilities open during the COVID-19 national emergency
SATURDAY, APRIL 18, 2020
Coronavirus at Smithfield pork plant: The untold story of America's biggest outbreak
REPORT
Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2
View ORCID ProfileJianzhong Shi1,*, View ORCID ProfileZhiyuan Wen1,*, View ORCID ProfileGongxun Zhong1,*, View ORCID ProfileHuanliang Yang1,*, View ORCID ProfileChong Wang1,*, View ORCID ProfileBaoying Huang2,*, Renqiang Liu1, Xijun He3, Lei Shuai1, Ziruo Sun1, Yubo Zhao1, View ORCID ProfilePeipei Liu2, Libin Liang1, Pengfei Cui1, Jinliang Wang1, View ORCID ProfileXianfeng Zhang3, Yuntao Guan3, View ORCID ProfileWenjie Tan2, View ORCID ProfileGuizhen Wu2,†, View ORCID ProfileHualan Chen1,†, View ORCID ProfileZhigao Bu1,3,† See all authors and affiliations
Science 29 May 2020: Vol. 368, Issue 6494, pp. 1016-1020 DOI: 10.1126/science.abb7015 Article Figures & Data Info & Metrics eLetters PDF Alternative hosts and model animals The severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) pandemic may have originated in bats, but how it made its way into humans is unknown. Because of its zoonotic origins, SARS-CoV-2 is unlikely to exclusively infect humans, so it would be valuable to have an animal model for drug and vaccine development. Shi et al. tested ferrets, as well as livestock and companion animals of humans, for their susceptibility to SARS-CoV-2 (see the Perspective by Lakdawala and Menachery). The authors found that SARS-CoV-2 infects the upper respiratory tracts of ferrets but is poorly transmissible between individuals. In cats, the virus replicated in the nose and throat and caused inflammatory pathology deeper in the respiratory tract, and airborne transmission did occur between pairs of cats. Dogs appeared not to support viral replication well and had low susceptibility to the virus, and pigs, chickens, and ducks were not susceptible to SARS-CoV-2.
Science, this issue p. 1016; see also p. 942
Abstract
Severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) causes the infectious disease COVID-19 (coronavirus disease 2019), which was first reported in Wuhan, China, in December 2019. Despite extensive efforts to control the disease, COVID-19 has now spread to more than 100 countries and caused a global pandemic. SARS-CoV-2 is thought to have originated in bats; however, the intermediate animal sources of the virus are unknown. In this study, we investigated the susceptibility of ferrets and animals in close contact with humans to SARS-CoV-2. We found that SARS-CoV-2 replicates poorly in dogs, pigs, chickens, and ducks, but ferrets and cats are permissive to infection. Additionally, cats are susceptible to airborne transmission. Our study provides insights into the animal models for SARS-CoV-2 and animal management for COVID-19 control.
snip...
In summary, we found that ferrets and cats are highly susceptible to SARS-CoV-2; dogs have low susceptibility; and pigs, chickens, and ducks are not susceptible to the virus. Unlike influenza viruses and the other SARS-coronavirus known to infect humans (SARS-CoV-1), which replicate in both the upper and lower respiratory tract of ferrets (20, 22–24, 26, 27), SARS-CoV-2 replicates only in the nasal turbinate, soft palate, and tonsils of ferrets. SARS-CoV-2 may also replicate in the digestive tract, as viral RNA was detected in the rectal swabs of the virus-infected ferrets, but virus was not detected in lung lobes, even after the ferrets were intratracheally inoculated with the virus. It remains unclear whether the virus causes more severe disease in male ferrets than in female ferrets, as has been observed among humans (13, 28).
Several studies have reported that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as its receptor to enter cells (3, 29–31). ACE2 is mainly expressed in type II pneumocytes and serous epithelial cells of tracheo-bronchial submucosal glands in ferrets (25). Ferrets and cats differ by only two amino acids in the SARS-CoV-2 spike-contacting regions of ACE2 (table S1); therefore, the underlying mechanism that prevents the replication of SARS-CoV-2 in the lower respiratory tract of ferrets remains to be investigated. The fact that SARS-CoV-2 replicates efficiently in the upper respiratory tract of ferrets makes them a candidate animal model for evaluating the efficacy of antiviral drugs or vaccines against COVID-19.
The cats we used in this study were outbred and were susceptible to SARS-CoV-2, which replicated efficiently and was transmissible to naïve cats. Cats in Wuhan have been reported to be seropositive for SARS-CoV-2 (32). Surveillance for SARS-CoV-2 in cats should be considered as an adjunct to elimination of COVID-19 in humans.
TUESDAY, JUNE 2, 2020
USDA APHIS Confirmation of COVID-19 in Pet Dog in New York
WEDNESDAY, APRIL 22, 2020
APHIS Confirmation of COVID-19 in Two Pet Cats in New York
Coronavirus can survive long exposure to high temperature, a threat to lab staff around world: paper
The new
can survive long exposure to high temperatures, according to an experiment by a team of French scientists.
Professor Remi Charrel and colleagues at the Aix-Marseille University in southern
heated the virus that causes Covid-19 to 60 degrees Celsius (140 Fahrenheit) for an hour and found that some strains were still able to replicate.
The scientists had to bring the temperature to almost boiling point to kill the virus completely, according to their non-peer-reviewed paper released on bioRxiv.org on Saturday. The results have implications for the safety of lab technicians working with the virus.
Coronavirus can survive long exposure to high temperature, a threat to lab staff around world: paper
The new coronavirus can survive long exposure to high temperatures, according to an experiment by a team of French scientists.
Professor Remi Charrel and colleagues at the Aix-Marseille University in southern France heated the virus that causes Covid-19 to 60 degrees Celsius (140 Fahrenheit) for an hour and found that some strains were still able to replicate.
The scientists had to bring the temperature to almost boiling point to kill the virus completely, according to their non-peer-reviewed paper released on bioRxiv.org on Saturday. The results have implications for the safety of lab technicians working with the virus.
MONDAY, JULY 6, 2020
It is Time to Address Airborne Transmission of COVID-19
tRump maga gop own this covid virus now in 2020, many dead and more will die due to their neglect and refusal to put human lives above party politics, and refusal to acknowledge science. now he is coming after your children in forcing them back into schools, while the pandemic is still in full force.
TUESDAY, JUNE 30, 2020
TRUMP COVID VIRUS 2020
Terry S. Singeltary Sr.

No comments:
Post a Comment